MYTH BUSTING 03: the TRUTH behind hair damage

A lot of hair damage happens here, on the surface.
When hair is damaged, the outer cuticle lifts and can become chipped and worn. The cells that normally overlap no longer lie flat. This leads to rough, dull strands which are prone to tangling—when the cuticle scales are not smooth, the strands catch and snag instead of gliding past each other. In turn, we tend to pull harder on that brush to get it through, doing even more damage which makes the hair shaft fragile and prone to splitting and breaking.
In addition, a damaged outer cuticle or damaged barrier leaves the inner layers of hair more vulnerable to damage. Think of the cuticle as the first line of defense for hair. Split ends happen because the longer hair is around, the more damage it has lived through. By the time it grows to the end of your style, it has seen a lot just by existing out in the world (think harsh UV rays or air pollution) and becomes very weathered. In extreme cases, this hair may have completely lost its cuticle and without the cuticle to hold the rod-like fibers in hair’s cortex intact, these fibers begin to unwind and separate, leading to what we know as split ends.
Hair is also sensitive to changes in hydration, or absorption of water. This behavior is influenced greatly by acidity and alkalinity (pH values). Alkaline (high pH) water—which is common for tap water across the US—causes hair to swell more, resulting in “opened” cuticle scales or a more permeable barrier to the hair’s interior. This makes hair susceptible to friction, frizz, and even its ability to withstand forces and heat, lowering its overall strength and resistance. 😳
the most extreme damage happens at the molecular level
When you add or remove color, perm, or relax hair, you break bonds that contribute to your hair's strength and elasticity. This happens at the inner-most layer of hair, in the cortex. During chemical shaping services like perms (curling) or relaxers (straightening), selectively breaking and re-forming disulfide bonds allows hair to be manipulated into new shapes. The chemical agents used in bleaching and color services allow stylists to strip or add color. Unfortunately, these chemicals can also break down the polypeptide chains responsible for hair’s core strength and elasticity during the process. With its structure so compromised, hair will look and feel not so good.
the little book of damage
Hair dyes often contain ammonia, which raises the pH of hair and causes the outer layer (the cuticle) to swell dramatically. The swelling of the cuticle allows the dye molecules to pass into the hair cortex where they will join together to color hair permanently. As with bleaching, peroxides are used—this time to oxidize the smaller dye molecules into bigger ones that cannot leave the cortex. Unfortunately, as we know, oxidizing agents are hard to control, and create collateral damage to hair’s disulfide and peptide bonds. Frequent dying of hair also can lead to excessive hair swelling which can result in permanent damage.
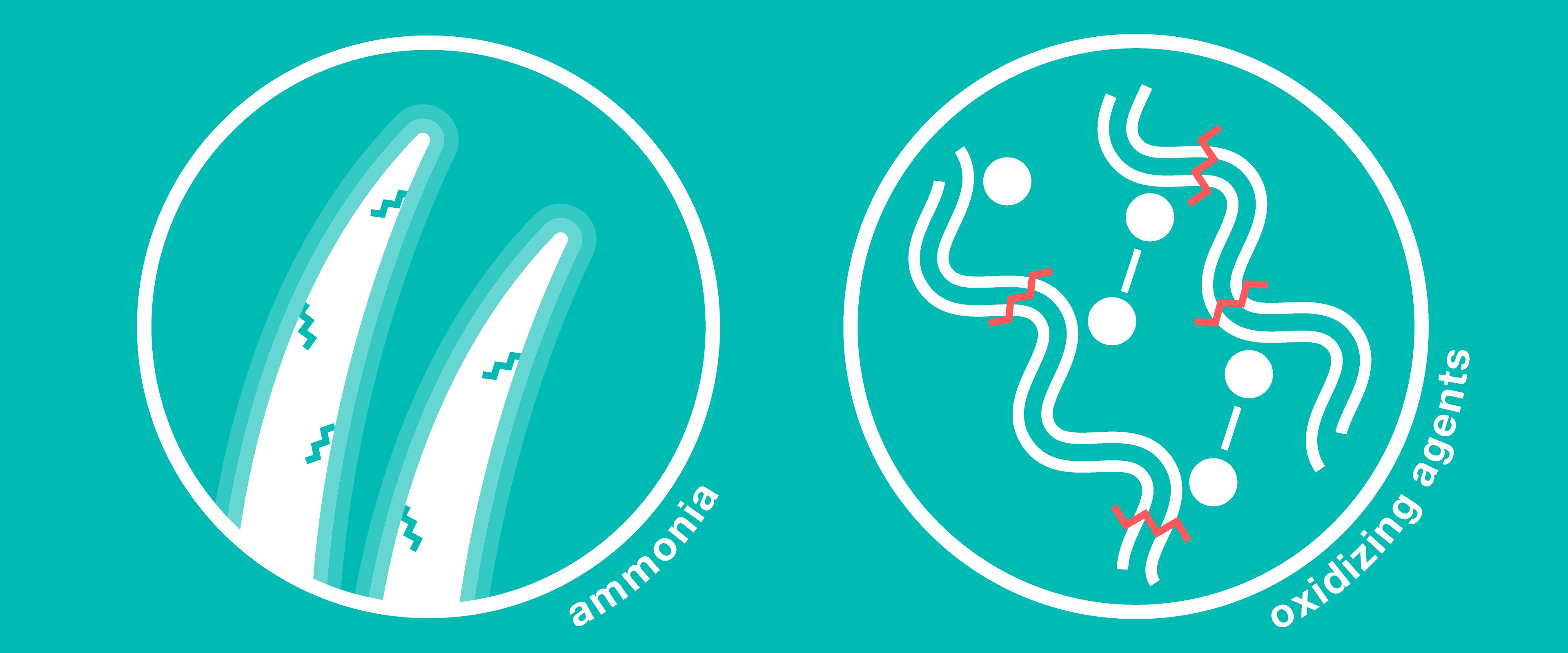
Perms use heat and/or chemicals to break down and reconfigure hair. In a standard cold perm, hair is put into curlers and a reducing agent, typically ammonium thioglycolate, is added. The thioglycolate compound breaks keratin’s disulfide bonds, separating them into individual cysteines. The keratin molecules adjust to the new shape of the curl, and cysteines are allowed to reconnect to disulfides in this shape, locking it in. This happens either slowly over time by the oxygen in air (which is why hair was traditionally not supposed to be washed for several days following a perm—Legally Blonde fans better be squealing right now. Once again, oxidizing agents run rampant and can cause incidental damage to the rest of hair’s structure. In addition, during the period of time when most of hair’s disulfides are broken at once, hair is more susceptible to damages. Finally, because the new shape of the hair isn’t its natural state, other bonds are broken to twist the shifted polypeptide chains, which are under strain to hold their new shape (imagine playing a particularly lively game of Twister and having to hold that pose indefinitely!) Hair may convert back to its natural state over time, but while strained it becomes weaker, and it sustains the damages incurred during the initial treatment.

Hair relaxers straighten hair chemically. They work by penetrating the cuticle and cortex of the hair to disrupt (reduce) and reconfigure the disulfide bonds between molecules much like during a perm, except with the opposite goal of loosening the natural curl pattern instead of creating one. Mechanical force (combing) and/or heat is applied to straighten the hair, followed by a neutralizing solution of hydrogen peroxide to reform the chemical bonds that will hold hair together in its new shape. As with perms, this process can leave hair weak, brittle, and prone to breakage—which is even worse when damaging chemicals like hydroxides are used instead of glycolates.

Flat-ironing hair causes the hydrogen bonds holding the keratin chains together to break and reform in a new position. This process allows the keratin chains to move around slightly and assume a temporary position that results in straightened hair. Hydrogen bonds are easily reset when hair is wet, which is why this kind of style won’t normally last past your next shampoo.
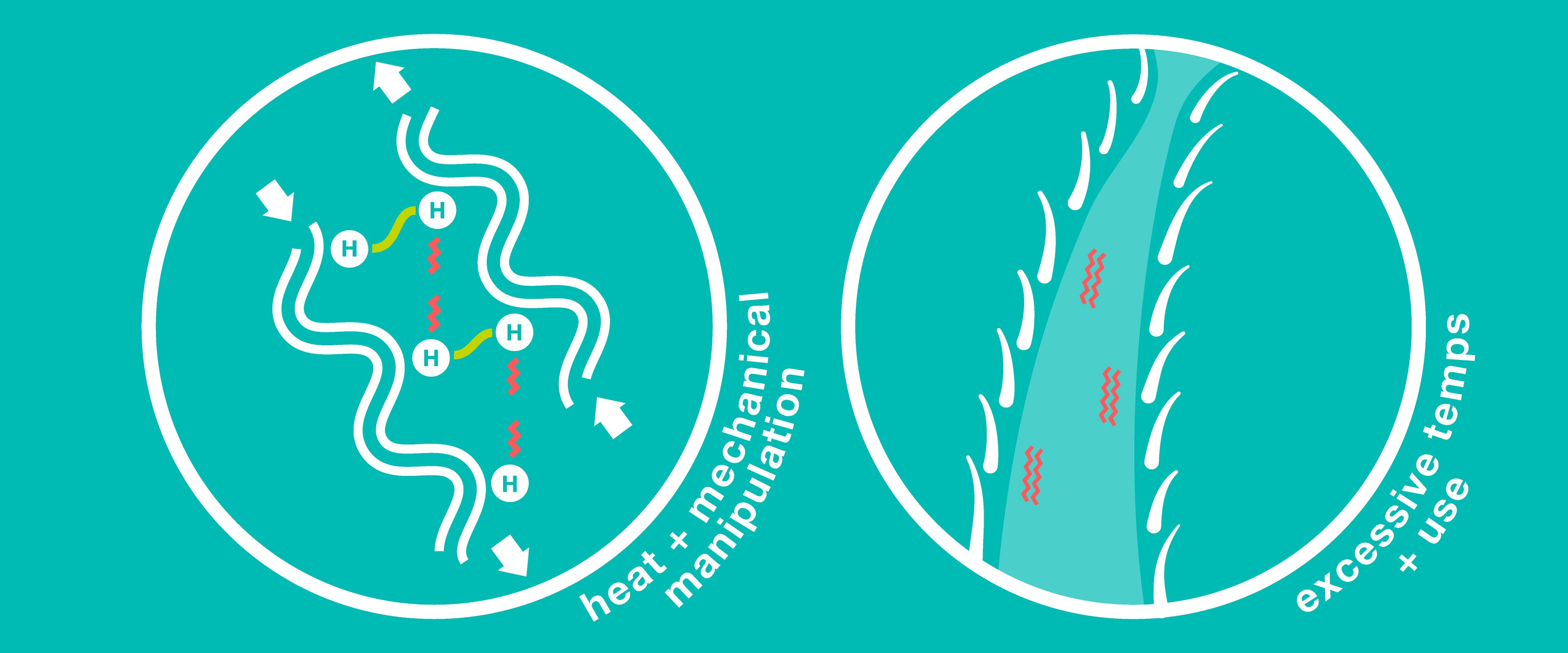
However, excessive heat degrades the cortex and damages keratin chains, resulting in dry, lackluster, or frizzy hair. Too much high temperature applied to very wet hair can also trap water molecules between keratin chains, causing bubble deformities to form in the hair shaft as these molecules are heated to their boiling point without a clear path to escape (think boiling tea kettle inside of each strand), leading to the deterioration of the cuticle and further hair breakage.
And don’t even get us started on UV and pollution… but that’s a Science Sunday for another time.
All this to say, we put our hair through a lot. And maybe, even more than you realized, is happening at a level we often can’t see post-styling or chemical service. The recurring theme for salon visits is the continual breaking or disruption of the polypeptide chains. These being the units responsible for hair’s strength and elasticity, it makes sense that when these are damaged hair can be left looking and feeling severely depleted.
But don’t panic! True renewal is possible, if you understand how and where the damage is happening and most importantly, how to get to it. By understanding damage at the scientific level, we were able to find the solve that understands the biology of your hair, so you’re left with strength, softness, smoothness, and bounce.


Leave a comment